Explosion Proof Switches & Sensors
Deeter Electronics designs, manufactures and sells a range of hazardous area products with ATEX and IECEx certification, including float switches, liquid level sensors and load cells. We can offer products with Ex d Explosion Proof approvals for use in hazardous areas without the need for an intrinsic safety barrier.
The Deeter Electronics hazardous area range also includes products which are suitable for use in Intrinsically Safe Systems with the use of a safety barrier.
Our Ex d range of Explosion Proof float switches and liquid level sensors with ATEX & IECEx certifications are suitable for use in explosive atmospheres, with these standards accepted in most regions worldwide.
-

DCS-IS Intrinsically Safe Capacitive Sensor
› view product
-

Explosion Proof Continuous Vertical Liquid Level Sensor with Integrated Display (LVCSi FP)
› view product
-

Explosion Proof Continuous Vertical Liquid Level Sensor (LVCS-FP)
› view product
-

Bespoke Explosion Proof Stainless Steel Float Switch (F/S-FP)
› view product
-

Vertically Mounted Hazardous Area Float Switch for Intrinsically Safe Systems (VFS-SA)
› view product
-
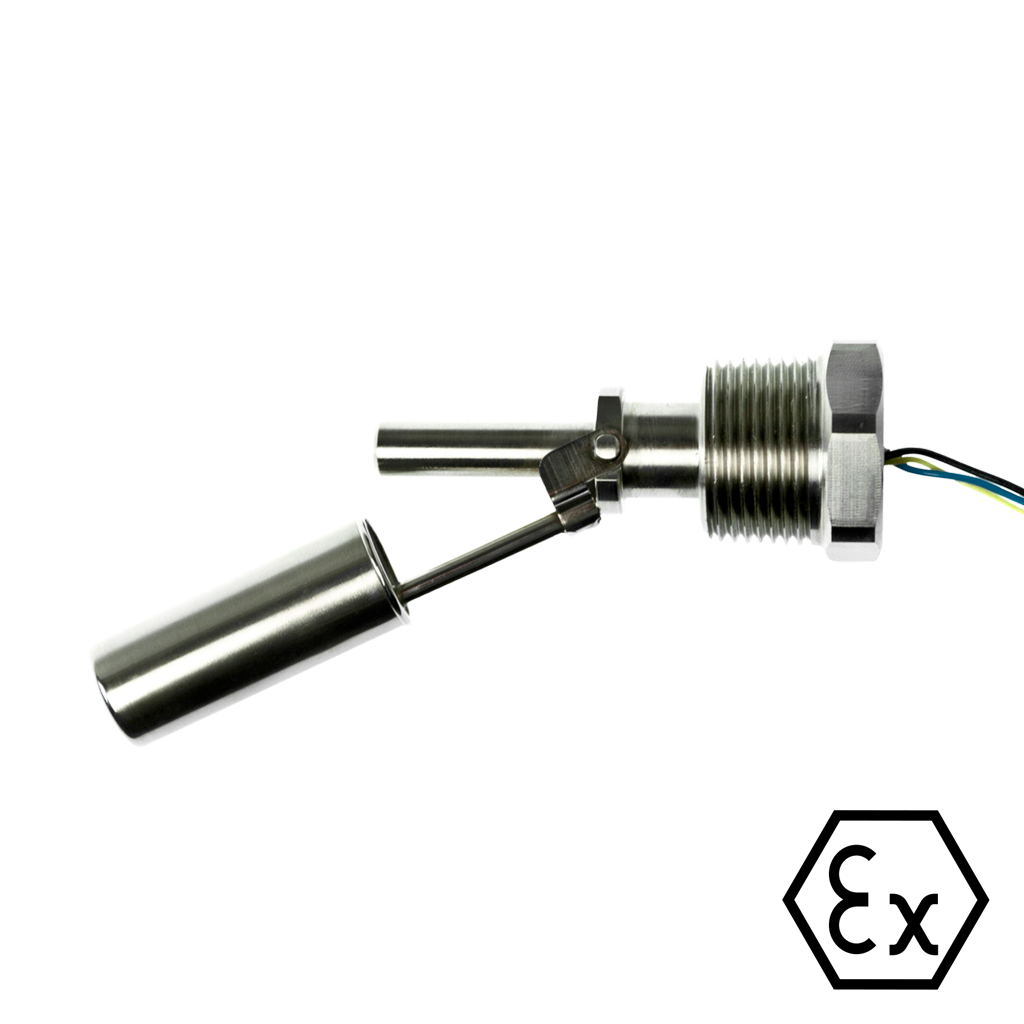
Horizontally Mounted Hazardous Area Float Switch for Intrinsically Safe Systems (HFS FP)
› view product
-

Explosion Proof Horizontal Float Switch (HFS Ex)
› view product
-

Explosion Proof Vertical Float Switch (VFS-FP)
› view product
-

BA304SG Hazardous Area Indicator
› view product
-

Marine Type Approved F/S-FP Series Stainless Steel Float Switch
› view product
-

RPS-409A-IS2 Intrinsically Safe Self-Contained Ultrasonic Sensor
Ranges: 4-40″, 6-80″
Intrinsically Safe
0 – 10 VDC Analog Output› view product
-

RPS-409A-IS3 Intrinsically Safe Self-Contained Ultrasonic Sensor
Ranges: 4-40″, 6-80″, 10-144″, 12-216″
Narrow Beam
0 – 10 VDC Analog Output› view product
-

Flameproof 4-20mA Loop Powered Indicators, Field Mounting
› view product
-

Type nA Non-Sparking 4-20mA Loop Powered Indicators, Field Mounting
› view product
-

Type nA Non-Sparking 4-20mA Loop Powered Indicators, Panel Mounting
› view product
-

Intrinsically Safe 4-20mA Loop Powered Indicators, Field Mounting
› view product
-

Intrinsically Safe 4-20mA Loop Powered Indicators, Panel Mounting
› view product
-

Intrinsically Safe 4-20mA Loop Powered Bargraph Indicator, Panel Mounting
› view product
Types of Hazardous Area Sensors, Switches and Equipment
- ATEX and IECEx Ex d Approved Explosion Proof Vertical Float Switch – a vertical magnetic float switch for control and indication of a liquid level while in a potentially explosive atmosphere.
- ATEX and IECEx Ex d Approved Explosion Proof Continuous Vertical Liquid Level Sensor – a magnetic float on a reed switch or Hall Effect sensor stem for control and indication of a liquid level while in a potentially explosive atmosphere.
- ATEX and IECEx Ex d Approved Explosion Proof Horizontal Float Switch – a horizontally mounting magnetic float switch for control and indication of a liquid level whilst in a potentially explosive atmosphere.
- Hazardous Area Float Switch for Intrinsically Safe Systems. A horizontal magnetic float switch for control and indication of a liquid level whilst in a potentially explosive atmosphere. Manufactured in 316L Stainless Steel, it is a horizontally mounted Liquid Level Sensor ideally suited to high temperature or pressure operation.
- Hazardous Area Ultrasonic Sensors. Sensors, controllers and Zener Diode safety barrier for use in intrinsic safety applications
- Load Cells. Weighing equipment with ATEX certification
